MEVPRO-1 is a Phase 3 randomized, open-label clinical trial to evaluate an investigational medicine taken orally by tablets called mevrometostat for men with metastatic castration-resistant prostate cancer (mCRPC) that has progressed after treatment with abiraterone acetate (Zytiga®).
MEVPRO-1 will investigate whether mevrometostat in combination with enzalutamide is superior to either enzalutamide or docetaxel in prolonging radiographic progression-free survival in the mCRPC setting.
Investigational mevrometostat has the potential to be the first-in-class EZH2 inhibitor for prostate cancer.
Epigenetic dysregulation is a hallmark of cancer cells.
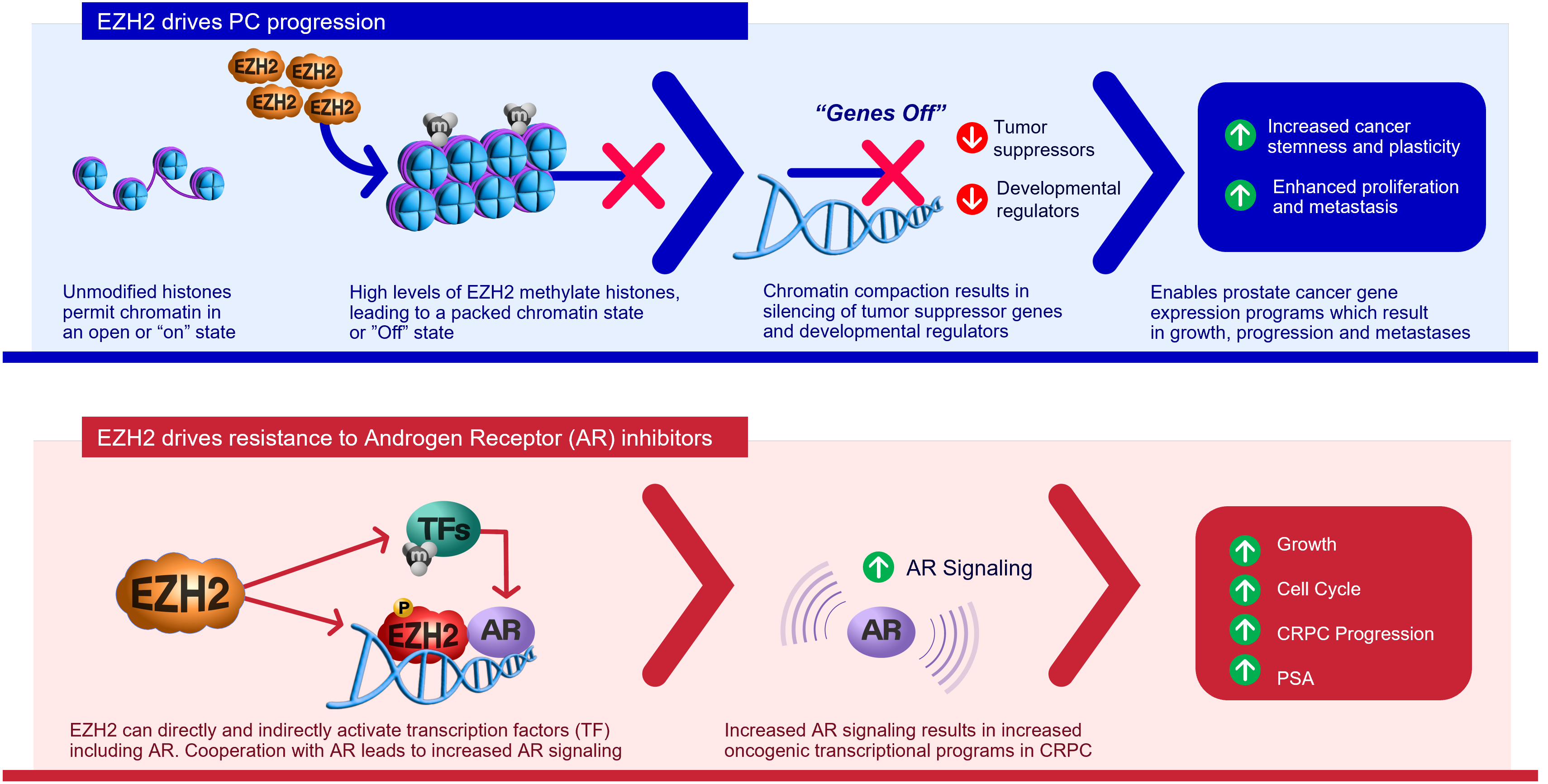
Enhancer of zeste homolog 2 (EZH2) is an epigenetic and histone methyltransferase regulator. Prostate cancer patients with higher expression of the EZH2 gene are associated with a worse prognosis.
EZH2 drives prostate cancer growth and treatment resistance in a number of ways including:
- Silencing tumor suppressor genes
- Activating androgen receptor (AR) genes
- Methylating non-histone targets
EZH2 is considered to be a novel target for cancer treatment.
Mevrometostat
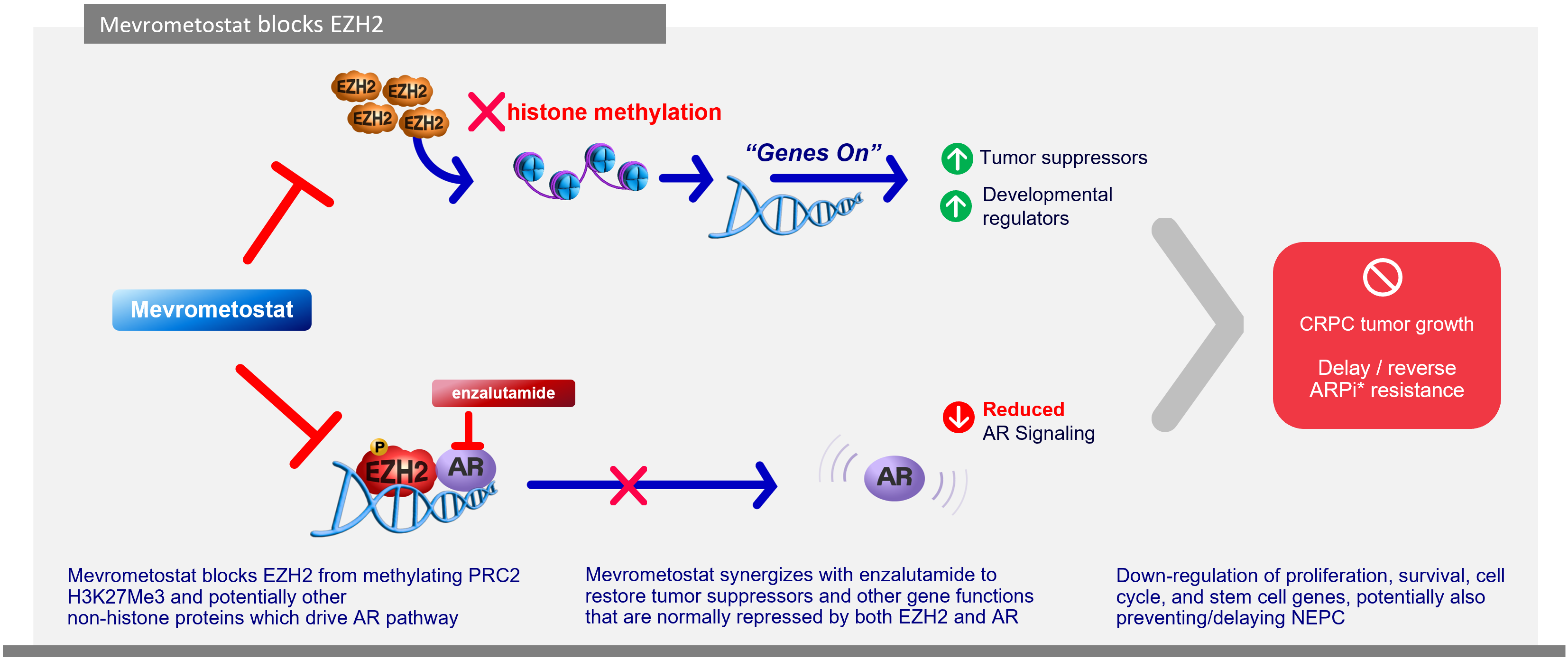
Investigational mevrometostat is a selective, and orally bioavailable inhibitor of the protein (EZH2).
In prostate cancer, mevrometostat has demonstrated synergistic effects when combined with enzalutamide that may delay or prevent the emergence of enzalutamide-resistant NEPC state.
Mevrometostat + enzalutamide may restore gene functions repressed by both EZH2 and androgen receptors, resulting in downregulation of proliferation, survival, and cell cycle progression.
Watch this video to learn more about mevrometostat
Mevrometostat is investigational and not approved by any health authority.
MEVPRO-1 is enrolling adult men (18+) who have:

Have histologically or cytologically confirmed adenocarcinoma of the prostate without small cell features

Have metastatic disease in bone documented on bone scan, or in soft tissue documented on CT/MRI scan

Have evidence of disease progression on or after treatment with at least 12 weeks of abiraterone acetate in the metastatic castration-sensitive prostate (mCSPC) cancer or first-line mCRPC setting
Approximately 600 participants will be randomly assigned on a 1:1 basis to:

Arm A:
Mevrometostat 875 mg BID + enzalutamide 160 mg QD

Arm B:
Study doctor’s choice of enzalutamide 160 mg QD OR docetaxel 75 mg/m2 IV every cycle (21 days) for up to a maximum of 10 cycles
Participants will receive investigational treatment until radiographic disease progression, unacceptable toxicity, protocol violation, withdrawal, or study termination. The length of participation in MEVPRO-1 will vary depending on how well participants tolerate the study treatment or time to disease progression.
The study includes a 28-day screening period, the treatment period, 28 to 35-day safety follow-up. The treatment period in the study will consist of cycles of 3 weeks (docetaxel group) or 4 weeks (enzalutamide groups). Participants receiving investigational mevrometostat and enzalutamide or enzalutamide alone will attend 2 study visits in Cycles 1 and 2, then 1 study visit per cycle thereafter. Participants receiving standard-of-care docetaxel will attend 1 study visit per cycle for up to 10 cycles.
Long-term safety follow-up will consist of visits every 12 weeks from the last dose until the study is complete, which may take many years, or until the participant withdraws consent.
Get started
Answer a 2-minute questionnaire and speak to a study representative.
A first step as you consider connecting with a Principal Investigator is to answer a 2-minute online questionnaire about your interest and willingness to be contacted. If your answers show the study might be a good fit for you and your patient, you may choose to have your contact information shared with a study clinic that you select for further discussion.
Get connected.
Your answers to these questions will only be linked to you if your responses indicate that you would like to be connected with a Principal Investigator and you choose to share your contact information with the study clinic. Pfizer study team members and our partners will have access to reports containing aggregated data that will not be directly linked back to you. Only the study staff can determine if your patient meets the study’s eligibility criteria and is able to enroll in the study.
People are often introduced to the world of clinical trials through conversations with their healthcare provider. As with anything new, there may be a number of questions, concerns, or misconceptions. Being their trusted healthcare provider, we appreciate you taking the time to have an open discussion with patients and their loved ones who may be interested in contributing to research.
Clinical trials maybe an option your patients will want to consider. While no benefit is guaranteed from an investigational medicine, your patients who participate in a clinical trial will contribute to the process of developing a potential new therapy.
If you would like more study-specific information to help inform conversations with potential participants, you can connect with a Pfizer trial site. Should any of your patients take part in the MEVPRO-1 clinical trial, they will remain under your medical care for all non-study-related needs and will continue to receive any benefits to which they are entitled.
To keep the line of communication open between you and your patients, feel free to follow-up with them to see if they randomized into this clinical trial and to keep in touch with them throughout their study journey.
Pfizer will give MEVPRO-1 clinical trial participants the option to access certain individual data, approximately 12 months after study completion. This allows participants to share data with their healthcare provider after their study participation concludes.
References
1. Srinageshwar B, Maiti P, Dunbar GL, Rossignol J. Role of Epigenetics in Stem Cell Proliferation and Differentiation: Implications for Treating Neurodegenerative Diseases. Int J Mol Sci. 2016;17(2):199.
2. Kaur P, Shankar E, Gupta S. EZH2-mediated development of therapeutic resistance in cancer. Cancer Lett. 2024;586:216706.
3. Ragavi R, Muthukumaran P, Nandagopal S, et al. Epigenetics regulation of prostate cancer: Biomarker and therapeutic potential. Urol Oncol. 2023;41(8):340-353.
4. Kung PP, Bingham P, Brooun A, et al. Kung PP, Bingham P, Brooun A, et al. Optimization of orally bioavailable enhancer of zeste homolog 2 (Ezh2) inhibitors using ligand and property-based design strategies: identification of development candidate (R)-5,8-dichloro-7-(Methoxy(oxetan-3-yl)methyl)-2-((4-methoxy-6-methyl-2-oxo-1,2-dihydropyridin-3-yl)methyl)-3,4-dihydroisoquinolin-1(2h)-one(PF-06821497).J Med Chem. 2018;61(3):650-665.
5. Park SH, Fong KW, Mong E, Martin MC, Schiltz GE, Yu J. Going beyond Polycomb: EZH2 functions in prostate cancer. Oncogene. 2021;40(39):5788-5798.
6. Du TQ, Liu R, Zhang Q, et al. EZH2 as a Prognostic Factor and Its Immune Implication with Molecular Characterization in Prostate Cancer: An Integrated Multi-Omics in Silico Analysis. Biomolecules. 2022;12(11):1617.
7. Bai Y, Zhang Z, Cheng L, et al. Inhibition of enhancer of zeste homolog 2 (EZH2) overcomes enzalutamide resistance in castration-resistant prostate cancer. J Biol Chem. 2019;294(25):9911-9923.
8. Kim J, Lee Y, Lu X, et al. Polycomb- and Methylation-Independent Roles of EZH2 as a Transcription Activator. Cell Rep. 2018;25(10):2808-2820.e4.
9. Xu K, Wu ZJ, Groner AC, et al. EZH2 oncogenic activity in castration-resistant prostate cancer cells is Polycomb-independent. Science. 2012;338(6113):1465-1469.
10. Nouruzi S, Tabrizian N, Zoubeidi A. Beyond Expression: Role of Phosphorylated Residues of EZH2 in Lineage Plasticity in Prostate Cancer. Endocrinology. 2023;164(4):bqad023.
11. Gautam N, Kaur M, Kaur S. Structural assembly of Polycomb group protein and Insight of EZH2 in cancer progression: A review. J Cancer Res Ther. 2021;17(2):311-326.
12. Data on file
13. Zhao JC, Yu J, Runkle C, et al. Cooperation between Polycomb and androgen receptor during oncogenic transformation. Genome Res. 2012;22(2):322-331.