The Be6A program is a series of clinical studies evaluating sigvotatug vedotin in multiple solid tumors expression integrin beta-6 (IB6)
Sigvotatug vedotin is a first-in-class investigational antibody-drug conjugate (ADC) designed to deliver the cytotoxic payload monomethyl auristatin E (MMAUE) to cells expressing IB6. IB6 is a surface cell receptor overexpressed in many solid tumors, such as non-small cell lung cancer (NSCLC), head and neck squamous cell carcinoma (HNSCC), esophageal cancer, and cutaneous squamous cell carcinoma.1
Data from the initial clinical study of sigvotatug vedotin demonstrates preliminary and encouraging antitumor activity and durability with a tolerable and manageable safety profile in heavily pretreated patients.2,3
Read on to learn more about sigvotatug vedotin and cancers that overexpress IB6 to see if your patients may qualify for enrolling studies.
Proposed mechanism of action
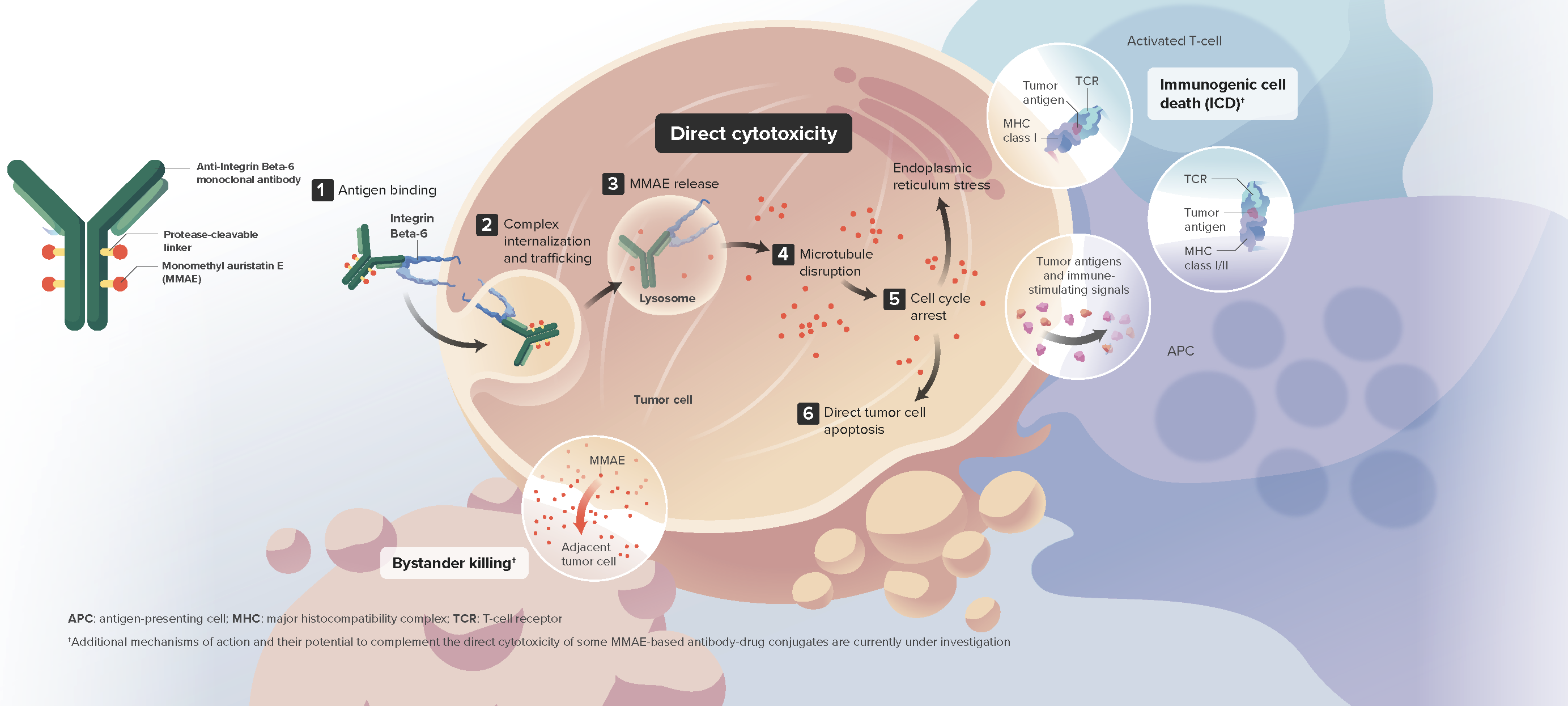
Sigvotatug Vedotin is thought to induce tumor cell death through:
- Direct cytotoxicity via preferential release of MMAE within target cells and subsequent apoptosis
- The bystander effect
- Immunogenic cell death4,5
About IB6-Overexpressing Cancers
IB6 is a member of the integrin family of proteins involved in cellular adhesion, motility, and cytokinesis. IB6 is expressed at low levels in normal adult epithelial tissues, but expression is induced by tissue injury due to its role in wound repair.6 High levels of IB6 expression have been demonstrated in different types of cancer1 and are associated with poor prognosis based on multiple retrospective analyses.7,8 Additional investigations suggest tumors may exploit the remodeling function associated with IB6 to promote invasiveness and metastasis.9,10
References
1. Lyon, RP, Jonas M, Frantz C et al. SGN-B6A: A new vedotin antibody-drug conjugate directed to integrin beta-6 for multiple carcinoma indications. Mol Cancer Ther. 2023;MCT02200817. doi:10.1158/1535-7163
2. Hollebecque A, Lopez J, Piha-Paul S, et al. A first-in-human of an integrin beta-6 targeted antibody-drug conjugate (ADC), SGN-B6A, in patients with advanced solid tumors: interim results of a phase 1 study (SGN-B6A-001). J Immunother Cancer. 2022;10(Suppl 2):A763
3. Hollebecque A, Lopez J, Piha-Paul, SA, et al. A first-in-human trial of an integrin beta-6 targeted antibody-drug conjugate (ADC), SGN-B6A, in patients with advanced solid tumors: Interim results of a Phase 1 study (SGNB6A-001). Poster presented at Society for Immunotherapy of Cancer Annual Meeting; Nov 8-12. 2022; Boston, MA.
4. Lyon R, Trang V, Gosnik JJ, et al. Abstract 1522: SGN-B6A induces immunogenic cell death as an additional mechanism of action. J ImmunoTher Cancer. 2022; 10(suppl 2). doi:10.1136/jitc-2022-SITC2022. 1186
5. Trang VH, Mazahreh R, Gosnik JJ, et al. Abstract 1522: SGN-B6A induces immunogenic cell death as an additional mechanism of action. Cancer Res. 2023;83(suppl 7):1522-1522. doi:10.1158/1538-7445.am2023-1522
6. Van Aarsen LA, Leone DR, Ho S, et al. Antibody-mediated blockade of integrin alpha v beta 6 inhibits tumor progression in vivo by transforming growth factor-beta-regulated mechanism. CancerRes. 2008;68(2):561-570.doi:10.1158/008-5472.CAN07-0245
7. Elayadi AN, Samli KN, Prudkin L, et al. A peptide selected by biopanning identifies the integrin alphabeta6 as a prognostic biomarker for nonsmall cell lung cancer. Cancer Res. 2007:67(12):5889-5895.
8. Elez E, Kocakova I, Hokler T, et al. Abituzumab combined with cetuximab plus irinotecan versus cetuximab plus irinotecan alone for patients with KRAS wild-type metastatic colorectal cancer: the randomised phase I/II POSEIDON trial. Ann Oncol. 2015;26(1):13-140. doi:10.1093/annonc/mdu474
9. Hamidi H, Ivaska J. Every step of the way: integrins in cancer progression and metastasis. Nat Rev Cancer. 2018:18(9):533-548. doi:10.1038/s41568-018-0038-z
10. Marsh D, Dickinson S, Neill GW, Marshall JF, Hart IR, Thomas GJ. Apha vbeta 6 integrin promotes the invasion of morphoeic basal cell carcinoma through stromal modulation. Cancer Res. 2008:68(9):3295-3303.doi:10.1158/0008-5472.CAN-08-0174